At first glance the world of medical device manufacturing may not seem much different from any other type of manufacturing: new products are researched and developed, materials are sourced, and the product is manufactured and sold, just like every other manufacturer.
However, as one of the most highly regulated industries in the business world, medical device manufacturers experience countless challenges on a daily basis. Product traceability, strict quality requirements and compliance with the government, and other regulatory requirements, are just some of the critical factors that can determine an organization’s success.
The COVID-19 pandemic put immense stress on medical device manufacturers. Not only did demand for certain products skyrocket, it practically disappeared for others. These manufacturers had to overcome disrupted supply chains and logistics, the operational impact of social distancing and safety measures, and a constantly changing reality—all while maintaining regulatory compliance, quality and production traceability, and more.
With so many new medical advancements being discovered, it is vital that medical device manufacturers remain highly responsive, continually streamlining time-to-market processes in order to retain market position.
How ERP can help medical device manufacturers
Manufacturing ERP refers to Enterprise Resource Planning (ERP) software and systems used to plan, manage and deliver specific functionalities that support manufacturers and manufacturing business operations. Choosing the right ERP system is essential if you want to achieve your business goals while also building a foundation for future operational improvements.
- Enterprise resource planning software for the medical device industry should be focused on helping companies meet government requirements. Companies should seek out systems for compliance assurance, quality management and supply management applications. ERP software for the medical device industry should also keep track of the following:
- Serial numbers
- Lot numbers
- Shelf life
- Accounting in different currencies
- Product data and cost analysis and management
- Quality management
- Production floor control
- Special production techniques, materials, and packaging for medical products force companies to keep stringent control over engineering and shop floor operations. The ability to create detailed work orders and access real-time data instantly is critical to production.
- Enterprise resource planning software for the medical device industry should be focused on helping companies meet government requirements. Companies should seek out systems for compliance assurance, quality management and supply management applications. ERP software for the medical device industry should also keep track of the following:
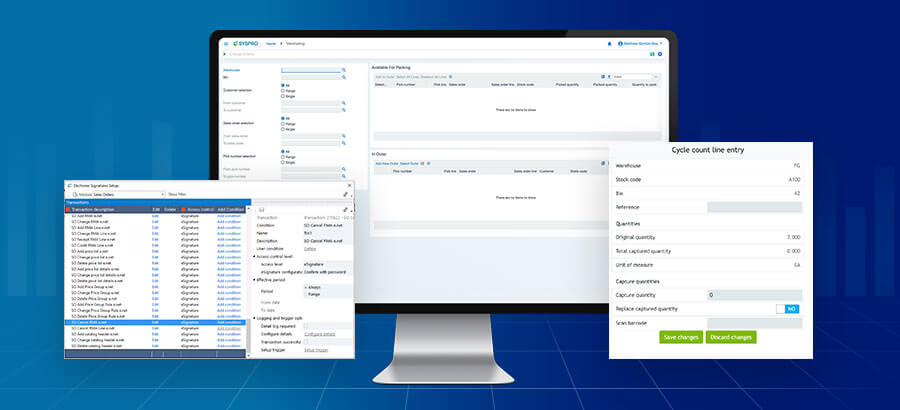
- The tracking and traceability ERP software provides will ensure that each stage of the manufacturing process comes with oversight. Easy to follow audit trails streamline the process and identify where, when, and how problems emerge. Recalls, while never an easy process, are swift and efficient thanks to ERP software that leverages automated lot and serial tracking to keep customers safe and businesses insulated from risk.
- Through automation, manufacturers can remove the manual tasks associated with logging data, such as billing and manufacturing execution. This cuts down on human error that causes most delays and downtime in manufacturing and sales cycles. While not necessarily a matter of regulatory compliance, the efficiency ERP software provides in this regard only strengthens the case for its adoption.
- Forward-looking organisations know that smart, enhanced ERP solutions are the key to meeting the extraordinary requirements of their uniquely demanding industry. And smart organisations know that they can adopt and leverage today’s advanced technologies to successfully manage ever-more-stringent regulations, dynamic product lifecycles, complex supply chains, multi-country compliance.
- SYSPRO’s inventory management functionality gives full transparency of materials, to ensure efficient storage of stock, meaning estimates become a thing of the past. Efficient production scheduling can help medical manufacturers respond more quickly to demand and enable the sector to compete in a competitive marketplace ensuring businesses remain productive and efficient at all times.
By investing in ERP, medical device manufacturers can streamline product development processes, to better ensure consistent standard compliance, affording unparalleled visibility and control over manufacturing, which in this sector, can literally mean the difference between life and death.